Embark on an enlightening journey into the realm of calorimetry with our comprehensive guide to student exploration calorimetry lab answers. This exploration delves into the intricacies of heat transfer, providing a profound understanding of the fundamental principles governing energy exchange.
Our meticulously crafted answers empower students to navigate the complexities of calorimetry experiments, enabling them to uncover the mysteries of specific heat capacity, temperature changes, and the fascinating applications of this field in diverse scientific disciplines.
1. Lab Overview: Student Exploration Calorimetry Lab Answers

The calorimetry lab experiment is designed to introduce students to the principles of calorimetry and the measurement of heat flow. The purpose of the lab is to determine the specific heat capacity of an unknown substance by measuring the temperature change of a known mass of water when the unknown substance is added to it.
2. Materials and Equipment
The following materials and equipment are required for the calorimetry lab:
- Calorimeter
- Thermometer
- Graduated cylinder
- Balance
- Unknown substance
- Water
The specific calorimeter used in the lab will vary depending on the resources available. However, all calorimeters are designed to measure the temperature change of a substance when heat is added or removed.
3. Experimental Procedures
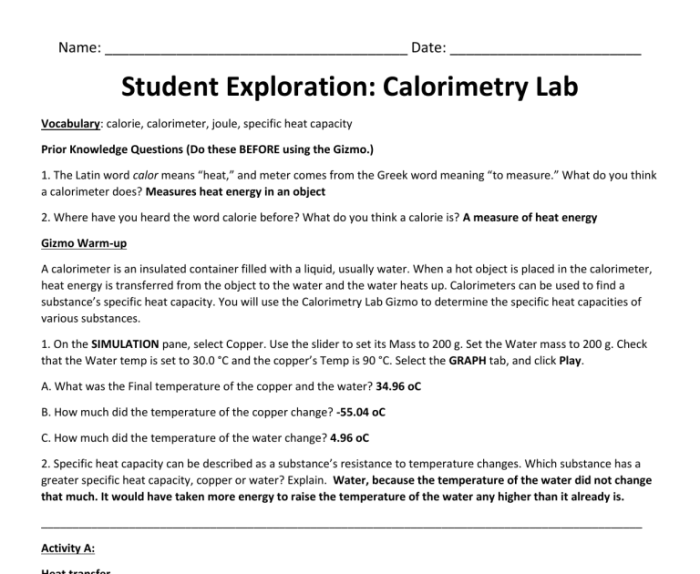
The step-by-step procedures for conducting the calorimetry lab are as follows:
- Measure the mass of the empty calorimeter and record it.
- Add a known mass of water to the calorimeter and record the temperature of the water.
- Add the unknown substance to the water and stir gently.
- Record the highest temperature reached by the water.
- Calculate the change in temperature of the water.
- Calculate the heat flow from the water to the unknown substance.
- Calculate the specific heat capacity of the unknown substance.
User Queries
What is the purpose of a calorimetry lab?
Calorimetry labs provide hands-on experience in measuring heat transfer and determining the specific heat capacity of substances.
How is heat flow calculated in calorimetry?
Heat flow is calculated using the formula Q = mcΔT, where Q represents heat flow, m is mass, c is specific heat capacity, and ΔT is the change in temperature.
What are some potential sources of error in calorimetry experiments?
Sources of error include heat loss to the environment, incomplete combustion, and inaccurate temperature measurements.